Confocal Microscopy: Difference between revisions
| Line 78: | Line 78: | ||
==Data Processing== | ==Data Processing== | ||
===Matlab Code=== | ===Matlab Code=== | ||
[[File:Fig_h.png| | [[File:Fig_h.png|x1400px|left|thumb]] | ||
==Experimental results== | ==Experimental results== | ||
Revision as of 16:36, 5 May 2022
Introduction
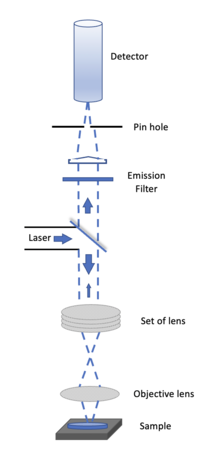
A Confocal Microscopy is an optical imaging technique for increasing optical resolution and contrast of a micrograph. It uses pinhole to block out all out of focus light to enhance optical resolution, very different from traditional wide-field fluorescence microscopes. To offset the block of out of focus lights, the light intensity is detected by a photomultiplier tube or avalanche photodiode, which transforms the light signal into an electrical one. We will try to build a Setup like this to enhance optical resolution and maybe get profile information about the sample.
Team Members
Wang Tingyu, Xue Rui, Yang Hengxing
Principles of confocal microscopy
The method of image formation in a confocal microscope is fundamentally different from that in a conventional fluorescence microscope , where the entire specimen is subjected to intense illumination from an incoherent lamp, and resulting image of secondary fluorescence emission can be viewed directly by eye or detector. This usually requires a thin, relatively transparent, sample but often results in out-of-focus blur that reduces resolution and specimen contrast. In contrast, the illumination in a confocal microscope is achieved by scanning focused beam from laser and across the specimen.
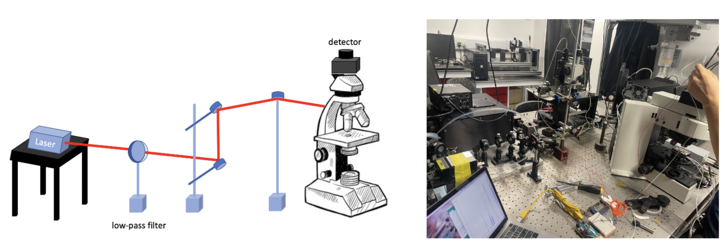
The confocal principle is diagrammatically presented in Figure 1. Coherent light emitted by the laser system (excitation source) pass through a low-pass filter that is situated in a conjugate plane (confocal) with a scanning point on the specimen and a pinhole aperture positioned in front of the detector (Si PIN photodiode). As the laser is reflected by a dichromatic mirror and scanned across the specimen in a defined focal plane, secondary fluorescence emitted from points on the specimen pass back through the dichromatic mirror and are focused as a confocal point at the pinhole aperture.
Set up
The confocal fluorescence microscope consists of multiple laser excitation sources, a scan head with optical and electronic components, electronic detector (Si PIN photodiode), and a computer for acquisition, processing, analysis, and display of images.
XYZ piezo scanner
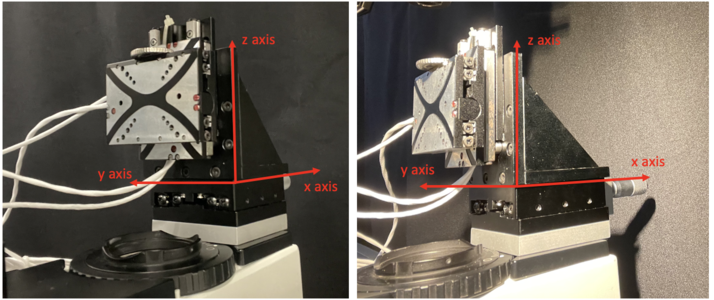
Piezo scanners are used for fast, high precision motion in one or more axes. In our project, we combine the micro PI stage controller with a moving stage on x-axis to realise three dimensional piezo scanning in our confocal microscopy.
Si PIN photodiode
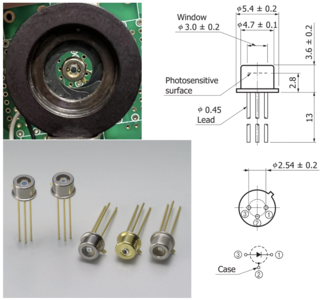
High-speed Si PIN photodiodes are designed for visible to near infrared light detection for they can efficiently convert light into an electrical current. The current is generated when photons are absorbed in the photodiode. These photodiodes provide wide-band characteristics at a low bias, making them suitable for optical communications. In this project, we choose S5973 series with a mini-lens type (S5973-01) that can be efficiently coupled with an optical fiber and a violet sensitivity enhanced type (S5973-02) ideal for violet laser detection.
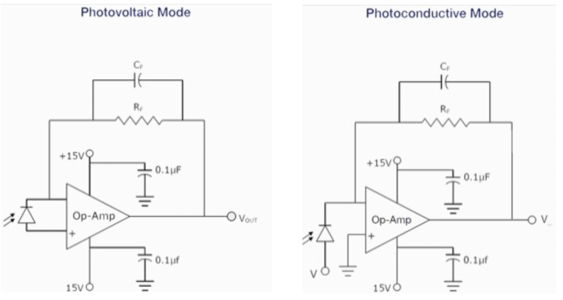
Photodiode has two working modes: solar cell mode (photovoltaic mode) and photoconductive mode. Solar cell mode is otherwise called as Zero Bias Mode. When a photodiode operates in low frequency applications and ultra-level light applications, this mode is preferred. When photodiode is irradiated by a flash of light, voltage is produced. The voltage produced will have a very small dynamic range and it has a non-linear characteristic. When photodiode is configured with OP-AMP in this mode, there will be a very less variation with temperature. (sketch diagram see link below). In the photoconductive mode, photodiode will act in reverse biased condition. Cathode will be positive and anode will be negative. When the reverse voltage increases, the width of the depletion layer also increases. Due to this the response time and junction capacitance will be reduced. Comparatively this mode of operation is fast and produces electronic noise.
Arduino UNO
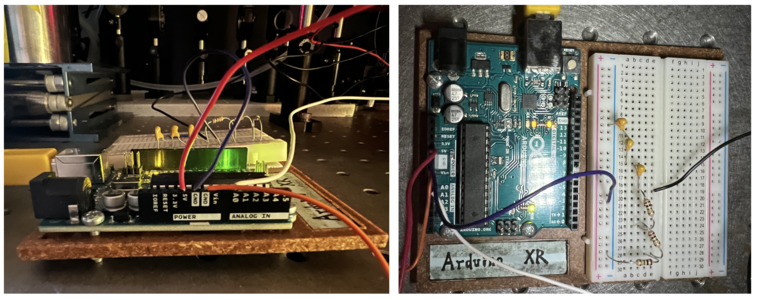
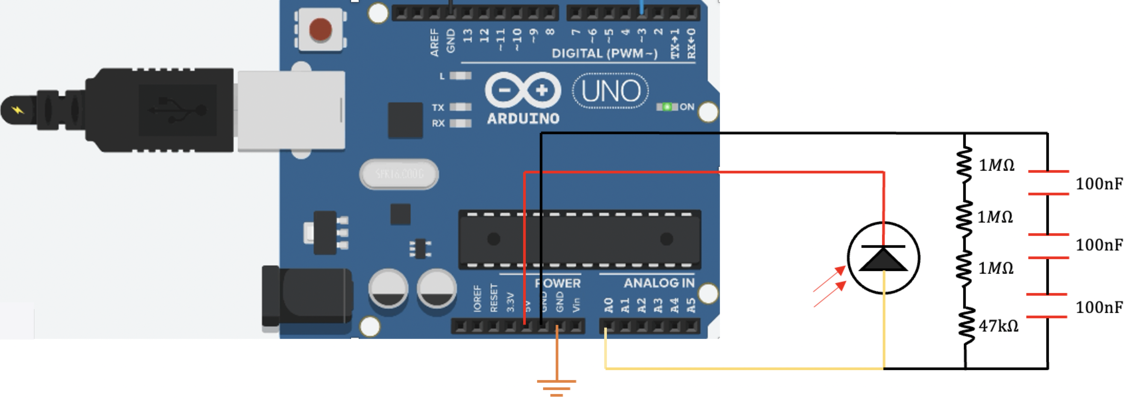
In our confocal set up, we use a 5V DC supply to inverse biase ( connect to the cathode of )the photodiode and operate it under a photoconductive mode. Some resistors are connect btw photodiode anode side and ground (GND) to amplify the signal from the photodiode and convert the photo current to an readable voltage. A 47K Ohm is first adopted in the circuit, later found out that this resistor is not big enough, then we added three more 1M Ohm resistors (3.047M Ohm in total). Then three 100 nF capacitors in series (100/3 nF in total )were connected btw photodiode anode side and ground (GND) to filter out the high frequency noises of photocurrent. Finally, We use an arduino analog input pin A0 to read out the voltage at the anode side of photodiode.
Also, in our photoconductive mode, we didn't use an OP-AMP, but connecting our voltage metter directly with our photodiode's anode.
Code
* Here is an Arduino sketch to read the output voltage of the photodiode detectors.
The output pin of the circuit is connected to analog pin 0 of the Arduino.
The data are read every 0.025 second in this example.
However, you can change the sampling rate by simply changing the argument of the delay() statement. *
#define inPin0 0
void setup(void) {
Serial.begin(9600);
Serial.println();
}
void loop(void) {
float pinRead0 = analogRead(inPin0);
Serial.println(pinRead0);
/* the minimum delay allowed is about 20, correspond
is about 20ms */}
delay(25);
}
Experimental process
Laser calibration
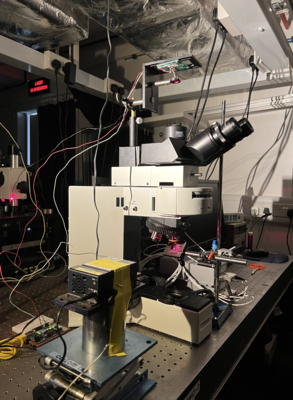
To realise the focus both on the focal plane of specimen and pinhole aperture, we built the set up shown in Figure.6. After the adjustment, we fix the height of the detector.
Measurement of coin sample
Step 1
Firstly, we managed to find the focus point within the region of 400um along z axis for each point on our coin by y-z micro PI stage controller.
Step 2
After that, we translated 100um on x-axis per step to find next focus point on z direction. We moved 10 steps in total so that the total distance along y-axis is 1000um.
Step 3
At last, we moved 100um on x-axis through tuning the spiral and measured the final set of data.
Data Processing
Matlab Code
Experimental results
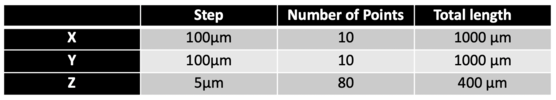
We tried to scan a 1mm*1mm area on the coin, and the area we focus is basically ‘E’ of word CENTS on a 50 cents Singapore dollar. Here are the scanning parameters of our experiment.
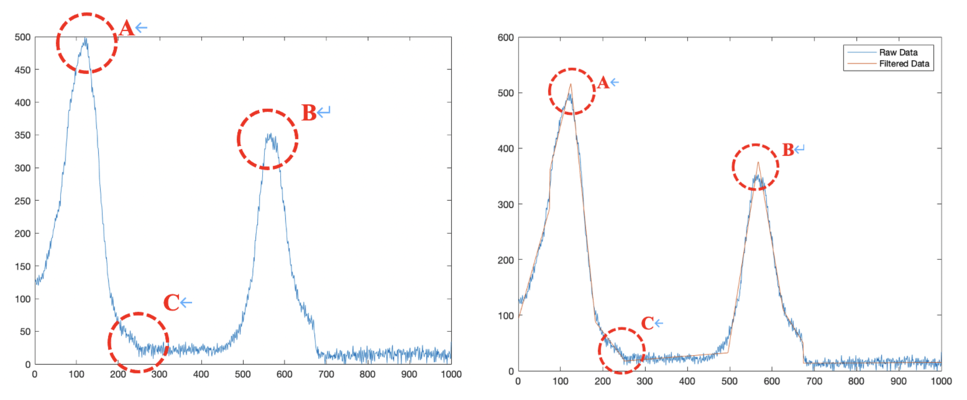
We used two piezo stage to move along y and z axis automatically, and a spiral micrometer to move the stage along x axis. Here we want to scan 10 points along x axis, 10 points along y axis and 80 points along z axis. Here is how we scan our sample: start from (x,y,z) the receiver will automatically scan 80 points when piezo moves upward along z axis (for scanning of each point piezo will take for about 85ms) and reach point (x,y,z+400), it will go pass the focused point of this certain (x,y) along the scanning of z axis, then piezo will move to point (x,y+100,z+400) and hold there for 5 seconds, then it will movie downward and reach point (x,y+100,z), it will pass through the focused point of this (x,y+400), and after that, it will move to point (x,y+200,z) and hold there still for 5 seconds. After 5 iterations of this, we will reach point (x,y+1000,z) and then we will use micrometer move the stage manually to point (x+100, y+1000,z) and repeat another 5 iterations, after moving 9 times we will reach point (x+1000, y+1000,z) finally. Figure below is the typical scanning pattern of two points (x,y,z+400) and (x,y+100,z+400), so we can find two corresponding maximum A&B in the plot, C is the point when piezo starts to hold still for 5 seconds.
By calculating length AC and BC, we can get height and intensity information about each (x, y). I use MATLAB function NF.m to filter noise and Figure.9 illustrates the filtered data.
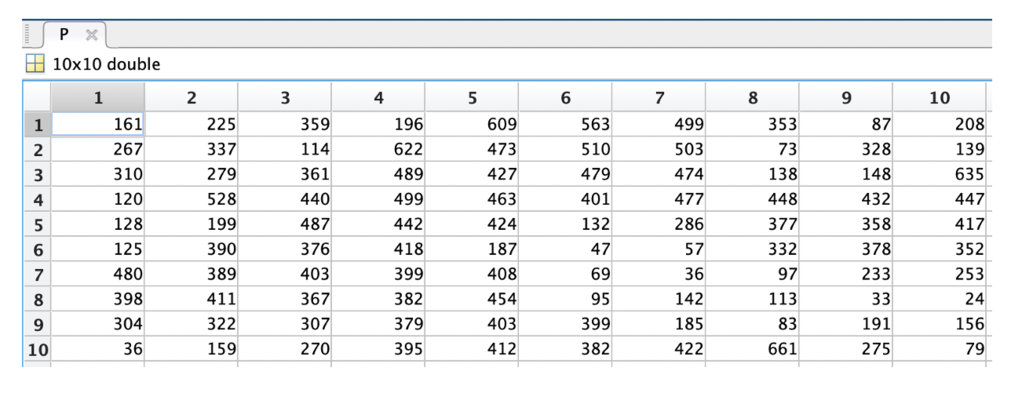
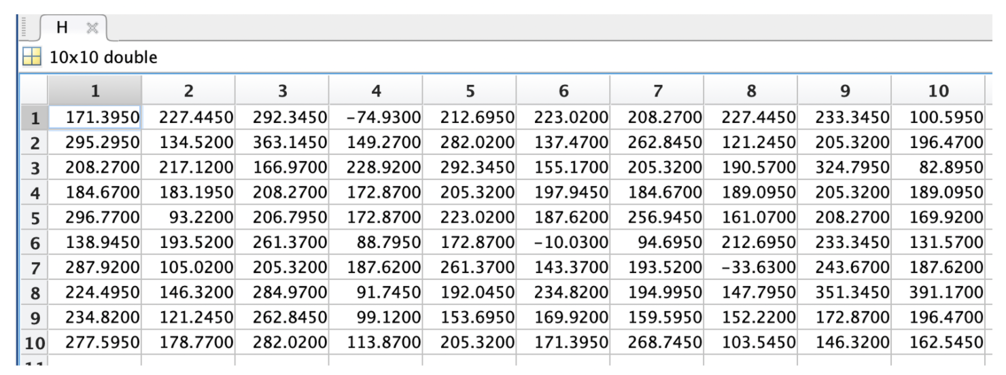
Dataphones are used to analyse the final intensity and height data. The intensity data of our sample are shown in Table 2.
The height data of our sample are shown in Table 3.
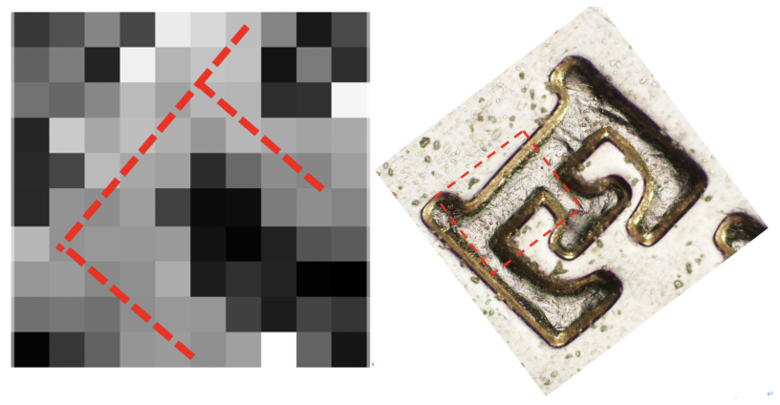
After that, we used the DrawSurf.m to draw Intensity data as illustrated in Figure 10. By comparing this result with the original "E" picture under an Optical microscope, we can find indeed this result looks like a letter "E".
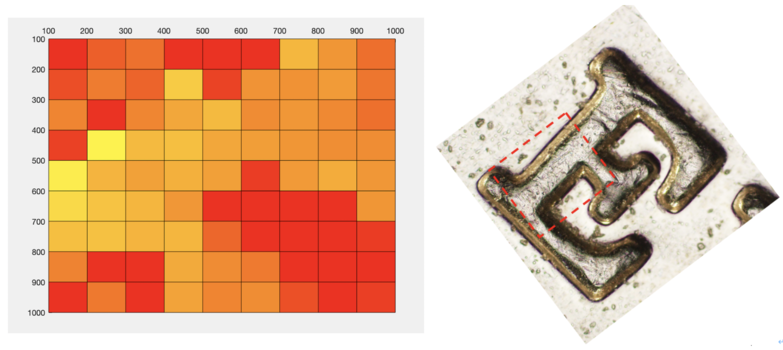
Besides, we also used the DrawTopo.m to draw Intensity data and Height data as illustrated in Figure.11. Height of the brighter orange region is bigger than the darker red region. By comparing this result with the original "E" picture under an Optical microscope, we can find indeed this result also looks like a letter "E".
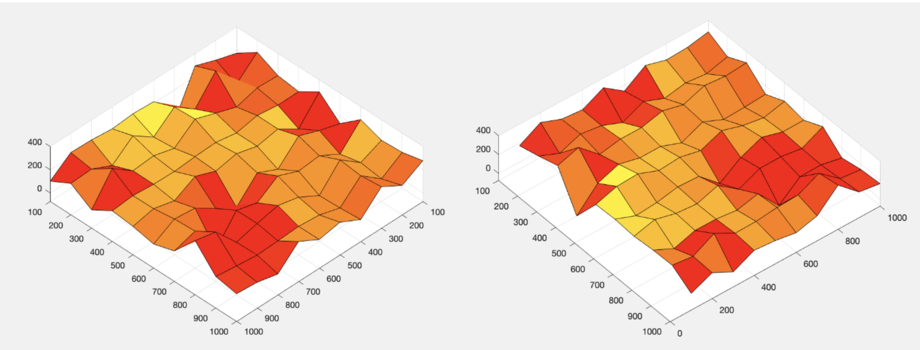
The final 3D results are shown in Figure 12. From that, it can be find that we have realised the three-dimensional imaging by confocal microscope.
Discussion
Future Works
Lab Location
S12-01-10/11 Nanomaterials Research Lab