Anti-glare LCD
Team members
Han Shixin(A0236394X)
Ming Xiaohan(A0236400W)
Zhang Yuanyuan(A0236393Y)
Idea
Glare is an undesirable lighting phenomenon. To suppress glare, optical films can be used. Based on the electro-optical characteristics of liquid crystals, the project aims to investigate the relationship between voltage and optical characteristics of liquid crystals. At the same time, the orientation and thickness of the liquid crystal are optimized simultaneously to obtain the design parameters of modulable liquid crystal anti-glare film, so that the liquid crystal has high diffraction efficiency at the reflecting end and low diffraction efficiency at the transmitting end.
Liquid crystal orientation technology
Liquid crystal orientation technology is the key to the preparation of liquid crystal instruments. The technology has been widely used for over a century, from 1911 to the present. The frictional orientation technique uses a material such as cotton lint or nylon, which is set in the direction of the frictional orientation and then rubbed against the orientation layer to give it a certain shape. Due to the anchoring effect of the orientation film, the molecules around the orientation layer will cause the liquid crystal layer molecules to show a certain regular arrangement, thus achieving the purpose of orientation. There are two theories of this technology: the "groove" theory and the "orientation of molecular chains on the surface of the oriented layer" theory. Groove theory refers to the higher density of grooves created by friction. From the point of view of low energy and high system stability, the liquid crystal molecules are aligned along the grooves and the intermolecular interactions result in a stable arrangement of the oriented layers.
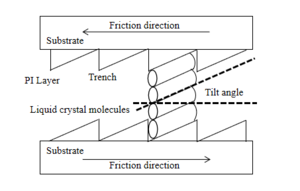
The theory of "surface molecular chain orientation" refers to the action between the liquid crystal molecules and the orientation layer. When the orientation layer is rubbed, special molecular chains emerge, which are extended when the molecules come into contact with it, and then extend to form a specific orientation. At present, neither of these two theories provides a perfect explanation of the mechanism of frictional orientation, and this method of contact orientation has a long history, but still requires further research. Later on, some non-contact orientation techniques emerged: ion beam orientation, oblique vapour deposition, light orientation techniques, etc.. One of these technologies, photo-orientation, refers to the physical or chemical reaction that occurs when light is directed into a material, due to its photosensitive properties, thus causing the liquid crystal molecules to achieve a specific pointing. Light-controlled orientation is widely used because it avoids static electricity, dust and other contamination and can be used to make multi-domain devices.
Theoretical foundations
2.1 Liquid Crystal 2.1.1 Introduction to liquid crystals Liquid crystal is a special state of matter between a liquid and a crystal, which has both the fluidity of a liquid and the anisotropy of a crystal [25] , and is known as the "fourth state of matter". In 1888, in the course of an experiment, Leinitzl discovered liquid crystals [26]. As an Austrian botanist, he discovered an interesting phenomenon when conducting experiments on the melting point of certain organic substances: when heating them up, at first the organic substances had a slightly white cloudy, opaque state and would appear in a number of different shades. After heating for some time, the temperature reached a certain value, the white cloudy disappeared and the liquid cleared up and became transparent. At the time, liquid crystals were not well known, but the phenomenon attracted a German physicist - Lehmann. He used a polarizing microscope with its own heating function to further observe this interesting phenomenon and found that these organic substances were birefringent in their opaque state, a special property of crystals. Based on the special properties of this substance, Lehmann gave it the name "Liquid Crystal". After liquid crystals were officially named, a number of interested scientists explored them further, but the experimental environment and knowledge were limited to theoretical research and no breakthroughs were made at the application level. As society moved forward and industrialization reached a certain level, liquid crystals came to the fore. 2.1.2 Classification of liquid crystals The classification criteria are different and the classification results for liquid crystals will be different. Liquid crystals can be classified as thermogenic liquid crystals or solvogenic liquid crystals, based on the conditions of formation of the liquid crystal state. Thermogenic liquid crystals are liquid crystals where the liquid crystal state exists within a certain temperature range, and solvogenic liquid crystals are liquid crystals where the liquid crystal state exists within a certain concentration range. There are also different results according to the classification of the molecular geometry of liquid crystals. The classification of liquid crystals differs according to the arrangement of the molecules, and can be divided into Nematic phase, Semotic phase and Chelesteric phase. The molecular arrangement of nematic phase liquid crystals is one-dimensional and ordered, but the center of gravity of the molecules is disordered. Unlike the nematic phase liquid crystals, the molecules of the proximal phase liquid crystals are all arranged in layers. The molecules in each layer of the near-crystalline A phase are arranged in one dimension and are ordered, but the center of gravity of the molecules is disordered; the molecules in each layer of the near-crystalline B phase are also arranged in one dimension and are ordered, and the center of gravity of the molecules is ordered; the molecules in the near-crystalline C phase are arranged in one dimension and are ordered, and the center of gravity of the molecules is disordered, but there is an angle between the arrangement of the molecules in the layer and the layer normal. The molecules of cholesteric liquid crystals are helically arranged, and on the surface perpendicular to the cross section of the helical axis, the liquid crystal molecules are arranged in one dimension and are ordered, but the center of gravity of the molecules is disordered.